MIL-STD vs. IEC Standards
Military Standard vs IEC Standard
 OY Elspecta-ab Member of US Militaria Forum
OY Elspecta-ab Member of US Militaria Forum
What are Military Standards?
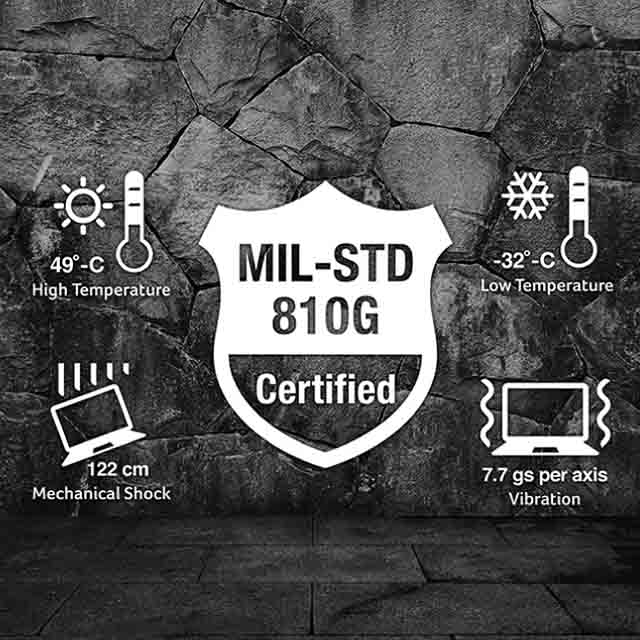
Military Standards (MIL-STD) are guidelines created by the U.S. Department of Defense to ensure equipment performs reliably in extreme military environments, including combat zones and harsh conditions.
What are IEC Standards?
IEC Standards are internationally recognized guidelines for testing the durability and reliability of electrical and electronic equipment, commonly used in industrial and transportation applications.
What is the Military Standard for Shock and Vibration?
MIL-STD-810 is the primary standard for testing shock and vibration, using Method 514 for vibration and Method 516 for shock, simulating real-world military scenarios.
What is the IEC Standard for Shock and Vibration?
IEC 60068 is the key standard for shock and vibration testing, including IEC 60068-2-27 for shock and IEC 60068-2-6 for vibration, focusing on industrial and transportation conditions.
OY Elspecta Lab Manager's for guidance on the requirement rationale use MIL-STD-464C, but depending on the equipment or system under test, would look to several derivative standards for additional performance and testing requirements:
MIL-STD-461G: Test RS-105 in this standard details the testing of sub-systems to the unclassified E1 waveform shown in Figure 1.
MIL-STD-188-125: Details pulse current injection (PCI) testing of fixed (MIL-STD-188-125-1) and mobile (MIL-STD-188-125-2) platforms to a separate, slower version of the E1 pulse.
MIL-STD-3023 HEMP protection for Aircraft
MIL-STD-4023 HEMP protection Ships
MIL-STD-2169 Classified HEMP testing environment for systems
API Spec Q1/Q2

API Spec Q1/Q2
Quality management for the oil and gas industry
API Specifications Q1 and Q2 are standards set by the American Petroleum Institute (API) for the certification of companies manufacturing components and providing services in the oil and gas production sector. The focus here is on training and reviewing quality standards and the quality assurance system. Risk assessment and risk management are taken into particular account. The standards are based on ISO 9001 and are supplemented by industry-specific requirements.
API Specification Q1:
Specification for Quality Management System Requirements for Manufacturing Organizations for the Petroleum and Natural Gas Industry
API Specification Q2:
Specification for Quality Management System Requirements for Service Supply Organizations for the Petroleum and Natural Gas Industries
We offer:
Auditing Services
Training Services
Auditing services
The specifications API Q1 and API Q2 are designed to address quality management systems for companies that manufacture products or provide manufacturing-related services according to a product specification for use in the oil and gas industry.
Declaration of impartiality - Committee for guaranteed impartiality
The OY Elspecta-ab has established processes to ensure that the certificating personnel always work impartially and independently. To guarantee the functionality of these processes, the certification authority works with a risk-based concept.
Check the permission of our company to use the API logo by entering the Labfinnland2018 code in this link: https://my.api.org/Account/Login

REACH (EN)
EU Chemicals Regulation: What does REACH reporting apply to?
REACH is the EU chemicals regulation and stands for “Registration, Evaluation, Authorisation and Restriction of Chemicals”. The REACH regulation is based on the European Regulation (EC) No. 1907/2006. Its purpose is to ensure that manufacturers and importers take responsibility for the safe handling of chemical substances and gradually replace harmful products.
“In principle, REACH applies to all chemical substances, i.e. not only those used in industrial processes, but also those found in everyday life, for example in cleaning agents, paints/lacquers, and products such as clothing, furniture and electrical appliances. Therefore, the regulation has an impact on most companies across the EU. (…) Manufacturer: If you manufacture chemicals to use yourself or to supply to others (including for export), you are likely to have to comply with some important obligations under REACH. Importer: If you buy something from outside the EU/EEA, you are likely to have to comply with some obligations under REACH. These may be individual chemicals, mixtures for resale, or finished products such as clothing, furniture, or plastic goods. Downstream users: most companies use chemicals, sometimes without even realizing it. Therefore, you need to check what your obligations are if you work with chemicals as part of your industrial or professional activities. You may have to fulfill some obligations under REACH. Source: European Chemicals Agency ECHA
e-Mark and E-Mark
The Europe has safety certification requirements formotor vehicle, components and systems that associated with security, and expressed as mark E and mark e.
The E-mark that approved by ECE derives from the regulations issued by Economic Commission of Europe. Recently ECE includes more than 50 countries, it also includesEastern Europe,Southern Europe such non-European countries apart from EU members, ECE regulation is recommended to the members but not mandatory standard. From the point of market demand, the ECE members usually willing to accept the test reports and certificates that comply with ECE regulations. The involved products are components and system units, but no the whole vehicle approval regulations.
The domestic common E mark certification products include tyre,safety glass,auto bulb, warning triangle, vehicle electronic products, etc. The testing organizations that perform E mark certification are usually the technical service organizations of ECE members, the license issuing agencies that issue E mark certification are government departments of ECE members. Different countries have their respective numbers:
E1 - Germany; E2 - France; E3 - Italy; E4 - Holland; E5 - Sweden; E6 - Belgium; E7 Hungary; E8 Czech; E9 Spain; E10 - Yugoslavia; E11 - Britain; E12 - Austria; Norway; Norway; Finland; Finland; Finland; Finland, Romania; Romania; Poland; E21 - Portuguese; E22 - Russia; E23 - Greece; E25 - Croatia; E26 - Slovenia; E27 - Slovakia; E28 - Belarus; E29 - Estonia; E31 - Bosnia and black; E37 - Turkey
The e mark that approved by EEC is the certification mark of whole motor vehicle, safety component and system that theEuropean Union Commission compels the members countries to use according to EU directives. The testing organizations have to be the technical service organizations of EU members, the license issuing agencies are government transport agencies of EU members. The approved products which acquire the e mark will be accepted by all EU members. Different countries have their respective numbers:
E1 - Germany; E2 - France; E3 - Italy; E4 - Holland; E5 - Sweden; E6 - Belgium; E9 Spain; E11 UK; E12 Austria; E13 - Luxemburg; E17 Finland; E18 - Denmark; Portugal; Greece; Ireland; Ireland
E-MARK sign: The e-Mark logo is divided into two forms, one is a rectangular outer frame, the other is a circular outer frame, representing different meanings.


The Finnish Transport Agency is responsible for traffic lanes in Finland and the overall development of the Finnish transport system. The Agency offers spatial data of roads, railways and waterways for viewing and downloading through WMS and WFS services. Also, A WMTS service providing nautical charts is under development.The Finnish Transport Agency has approved various laboratories to conduct EMC tests and Manufacturer Conformity of Production (COP) Audit Introduction for the issuance of the E/e-Mark car certificate throughout Europe, among these laboratories are Midcert, OY Elspecta & Emitech Group.
ECE R10 TYPE APPROVAL OR HOMOLOGATION
After ECE R10 EMC tests are done and PASS test report issued, Type Approval Certificaition or Homologation (E-marking) can be also issued for the product. In this step, ECE R10 test report, technical documents of the product, Conformity of Production (CoP) inspection report/certificate, quality documents (like valid ISO 9001 certificate) of the manufacturing or/and distributor company are checked by a third party and authorized agency. They are also called designated technical service. If all product and company documents are issued and submitted properly, E mark including a unique number can be affixed or labeled on the product.
An approval number is assigned to each type approved. The type approval number consists of 4 sections. Each section shall be separated by the ‘*’ character.
Section 1: The capital letter ‘E’ followed by the distinguishing number of the Contracting Party which has granted the type approval.
Section 2: The number of the relevant UN Regulation, followed by the letter ‘R’, successively followed by:
(a) Two digits (with leading zeros as applicable) indicating the series of amendments incorporating the technical provisions of the UN Regulation applied to the approval (00 for the UN Regulation in its original form);
(b) A slash and two digits (with leading zeros as applicable) indicating the number of supplement to the series of amendments applied to the approval (00 for the series of amendments in its original form);
(c) A slash and one or two character(s) indicating the implementing stage, if applicable.
Section 3: A four-digit sequential number (with leading zeros as applicable). The sequence shall start from 0001.
Section 4: A two-digit sequential number (with leading zeros if applicable) to denote the extension. The sequence shall start from 00.